What is Thermal Conductivity of Materials Explained with Examples and Applications
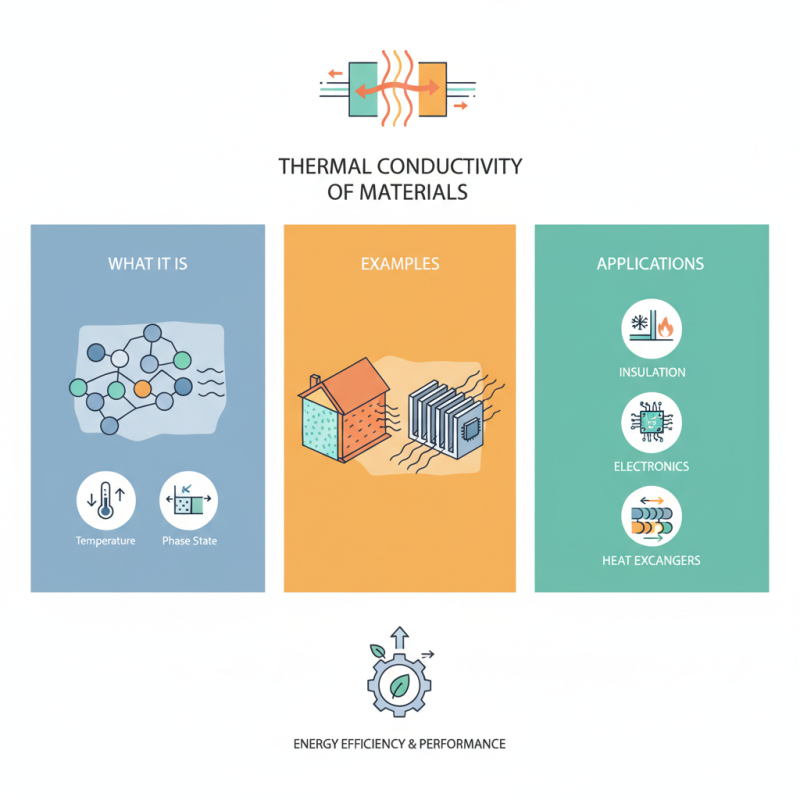
The thermal conductivity of materials is a fundamental property that plays a crucial role in the design and application of various engineering solutions. It refers to the ability of a material to conduct heat and is determined by its characteristics such as molecular structure, temperature, and phase state. Understanding the thermal conductivity of materials is essential for optimizing energy efficiency in systems such as insulation, electronics, and heat exchangers.
In practical applications, materials with diverse thermal conductivities are utilized in a wide range of industries—from construction and manufacturing to electronics and aerospace. For example, insulative materials like foam are designed to minimize thermal conductivity to maintain temperature stability, while metals like copper, known for their high thermal conductivity, are used in heat sinks and conductive pathways.
This article will delve deeper into the concept of thermal conductivity of materials, providing examples that illustrate its significance and exploring various applications that highlight how material selection based on thermal properties can lead to enhanced performance and energy conservation across different fields. Through this understanding, engineers and designers can make informed decisions that leverage the thermal properties of materials to address specific challenges and requirements.
Definition of Thermal Conductivity and Its Significance
Thermal conductivity is a fundamental property of materials that quantifies their ability to conduct heat. It is defined as the amount of heat that passes through a unit thickness of a material per unit time when there is a temperature gradient across it. This property is crucial in various fields such as engineering, construction, and manufacturing, as it directly influences energy efficiency and thermal performance. Materials with high thermal conductivity, like metals, are excellent conductors of heat, making them ideal for applications such as heat exchangers and cooking utensils. In contrast, materials with low thermal conductivity, such as insulation substances, are necessary for reducing heat transfer, thus promoting energy conservation in buildings and appliances.
Understanding thermal conductivity is essential for material selection in both product development and engineering design. In construction, for instance, choosing materials with appropriate thermal conductivity can significantly affect a building's heating and cooling requirements, leading to sustainable energy use. Likewise, in the manufacture of electronic devices, managing heat generation and dissipation is critical for performance and reliability. Thus, thermal conductivity not only plays a significant role in improving the functionality of products but also in addressing environmental concerns by enhancing energy efficiency across various applications.
Factors Influencing Thermal Conductivity in Materials
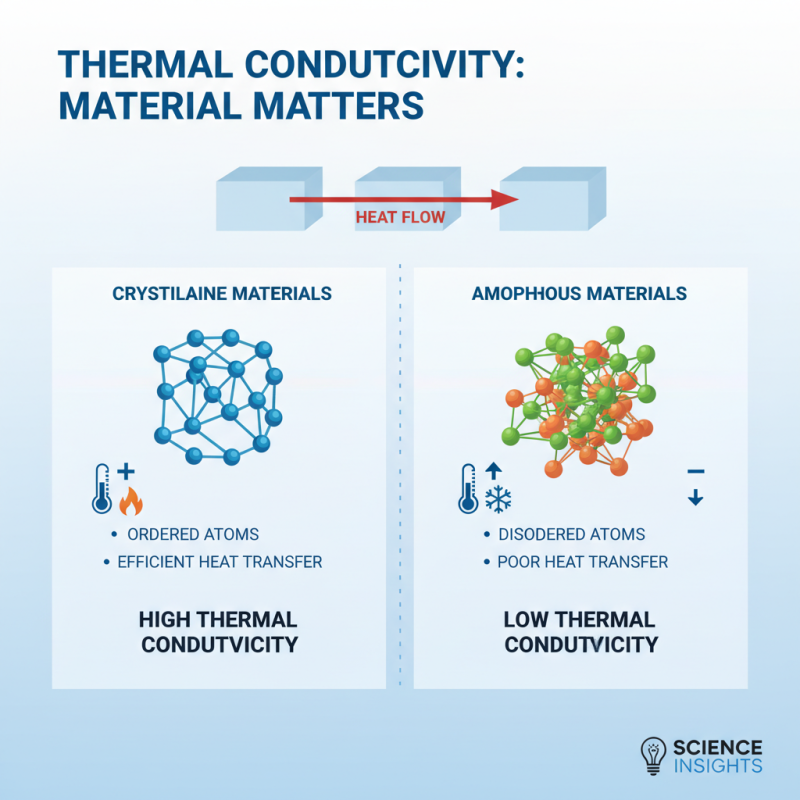
Thermal conductivity is a crucial property of materials that determines how well they can conduct heat. Several factors influence this characteristic, significantly impacting the selection and application of materials in various fields. One primary factor is the material's structure. For instance, crystalline materials tend to have higher thermal conductivity due to the orderly arrangement of atoms, which facilitates efficient heat transfer. In contrast, amorphous materials, which lack such structured organization, typically demonstrate lower thermal conductivity.
Another influential factor is the temperature of the material. As temperature increases, the atomic vibrations within a material also rise, which can enhance thermal conductivity to a certain extent. However, for some materials, high temperatures may lead to increased phonon scattering, reducing their overall ability to conduct heat. Additionally, the presence of impurities and defects within a material can alter its thermal conductivity. Impurities disrupt the orderly flow of heat, often resulting in decreased thermal performance. Understanding these factors is essential for engineers and designers when selecting materials for applications involving temperature regulation, insulation, and energy efficiency.
Common Examples of Materials with High and Low Thermal Conductivity
Thermal conductivity is a crucial property that defines how well materials can conduct heat, significantly impacting various applications across industries. For instance, metals such as copper and aluminum exhibit high thermal conductivity, making them ideal for applications such as heat exchangers and thermal management systems. Copper has a thermal conductivity of approximately 400 W/m·K, while aluminum follows closely at about 205 W/m·K. This high conductivity allows for efficient heat transfer, which is essential in electronics cooling systems and cooking utensils.
On the other end of the spectrum, insulative materials like rubber, wood, and certain plastics display low thermal conductivity. For example, rubber typically has a thermal conductivity of around 0.1 W/m·K, while wood can range from 0.1 to 0.2 W/m·K depending on its density. These materials are widely used in construction and manufacturing to prevent heat loss and improve energy efficiency in buildings. The distinct differences between these materials illustrate their suitability across various applications, influencing energy consumption and operational efficiency in numerous sectors.
Applications of Thermal Conductivity in Various Industries
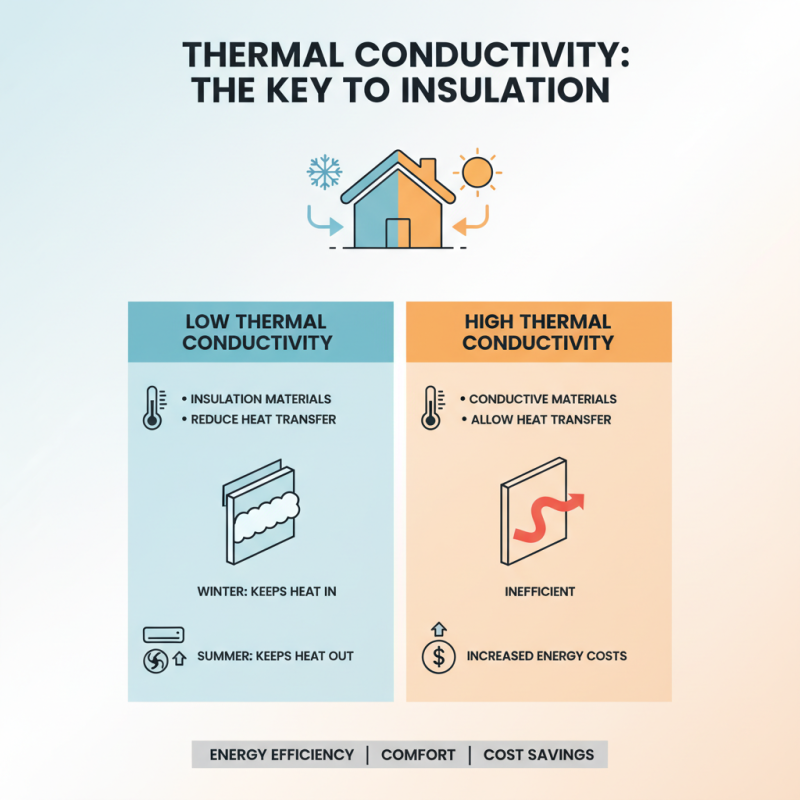
Thermal conductivity is a critical property of materials that plays an essential role across various industries. In construction, for example, materials with low thermal conductivity are favored for insulation purposes. This helps in maintaining energy efficiency within buildings, as insulating materials reduce heat loss during the winter and minimize heat gain in the summer. The effectiveness of insulating materials directly impacts heating and cooling costs, making thermal conductivity a vital consideration in architectural design and material selection.
In the electronics sector, thermal conductivity is crucial for managing heat dissipation in devices such as computers and smartphones. High thermal conductivity materials are employed to ensure efficient heat transfer away from sensitive components, which helps in maintaining optimal operating temperatures and enhancing the longevity of the devices. Additionally, in the automotive industry, managing thermal efficiency through the selection of materials with specific thermal conductivity values allows for improved performance and safety in vehicles. These applications highlight the significance of understanding thermal conductivity in developing innovative solutions that enhance energy efficiency and product reliability across various domains.
Measuring Thermal Conductivity: Methods and Techniques
Measuring thermal conductivity is essential for understanding a material’s ability to conduct heat, and several techniques have been developed to obtain accurate measurements. One of the most commonly used methods is the steady-state technique, where a constant heat flow is maintained through the material. By measuring the temperature difference across the material and the applied heat, one can calculate the thermal conductivity using Fourier’s Law. This approach is particularly useful for materials that exhibit consistent thermal properties under stable conditions.
Another method is the transient or dynamic technique, which involves applying a short heat pulse to the material and observing the resulting temperature changes over time. The most widely recognized transient method is the laser flash analysis, where a brief laser pulse heats one side of the sample while a detector measures the temperature rise on the opposite side. This allows for rapid assessment of thermal conductivity in a variety of materials, including solids, liquids, and even gases. These techniques not only enhance our understanding of thermal management in industrial applications but also aid in the development of new materials engineered for specific thermal performance.
Related Posts
-

7 Best Materials with Exceptional Thermal Conductivity You Should Know
-
Understanding the Impact of Thermal Conductivity on Material Selection in Engineering
-

Understanding the Thermal Conductivity of Materials: Key Factors and Measurements
-

How to Optimize Your Energy Production with Effective PV Monitoring Strategies
-

Top 10 Temperature Probe Sensors for Accurate Cooking and Baking
-

2025 Top Contact Temperature Sensor Trends and Innovations You Need to Know







